Technology
Executive Summary
EyeCell is focused on development of microcurrent devices
EyeCell is focused on the development and sales of microcurrent devices for treating eye related diseases and disorders The company has developed and patented eight product lines:
- Current of injury microcurrent devices for healing and pain management (3)
- Current of injury microcurrent bandages.
- Stem cell regeneration microcurrent device for regeneration, healing
and improvement of blood flow. - Anti-angiogenic (stops over blood supply) signaling microcurrent device.
- Stem cell growth factor cocktail compositions to be injected.
Micropump for sustained delivery of eye repair agents over time. - Eye health vitamins for
post procedure healing. - Microcurrent acupuncture point positioned eye patch.
The company has FDA 510K market clearance on three current of injury microcurrent products and one CE Mark for a wireless microcurrent stimulator working with selected vendors for OEM private label manufacturing.
The EyeCell microcurrent devices are designed to provide healing of injuries, reduce inflammation, reduce pain and risk of infection.
EyeCell collaborative researchers have treated nearly 3000 patients to date with 90% of them demonstrating improvement directly related to
Eye Regeneration by Bioelectric Controlled Protein Expression and Stem Cell Based Composition Micro Infusion
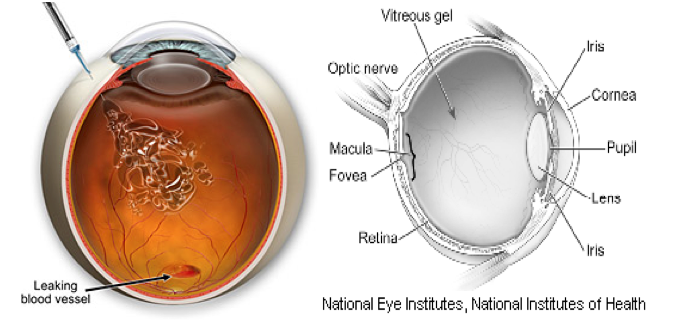
THE PROBLEMS
Wet Macular Degeneration
More than 20 million people worldwide suffer
Dry Macular Degeneration
Retinal Degenerative Diseases
Diabetic retinopathy
Solution
EyeCell’s treatment armamentarium is designed to (investigational use only):
Seal off leaky blood vessels.
- Reduce inflammation.
- Regenerate tissues healing.
- Stop over blood supply.
- Increase oxygenated blood supply when needed.
- Reduce infection risk.
- Reduce Pain.
Seal off leaky blood vessels.
- Reduce inflammation.
- Regenerate tissues healing.
- Stop over blood supply.
- Increase oxygenated blood supply when needed.
- Reduce infection risk.
- Reduce Pain.
Edward C. Kondrot, MD, our Executive Vice President of Clinical Studies and Co-Founder summarized three studies on the use of microcurrent for AMD:
1. A two-year study (1983 to 1985) involving 114 patients, conducted by Grace Halloran,
- Eighteen patients had macular degeneration; 16 improved.
- Seventy-eight patients had retinitis pigmentosa; 62 showed improvement.
- Eighteen patients had other various retinopathies; 16 improved.
- Of the patients who did not demonstrate any improvement, 14 stayed the same (although they otherwise would have been expected to lose vision); two continued to lose vision, although only slightly.
1.
- Seventy-eight percent of the eyes showed from 1-9 lines of improvement in reading of a visual acuity chart.
- Over 50 percent improved from 2-9 lines of improvement.
- In the study, two patients suffered from retinal vein occlusion and swelling of the macula. Both had
dramatic improvement in vision.
1.
- Of the 120 patients treated, 83 percent showed improvement of greater than or equal to two lines of visual acuity in one or both eyes.
Mechanisms of Action:
Primary = Delivers stem cells growth factors directly to wound site and stimulates the over expression of the stem cell homing signal protein SDF-1 which is also a powerful arteriogenic agen.
2. Multiples recruited and delivered stem cells to greater quantities at wound site.
3. Increases blood flow at wound site 3X (from delivering and recruiting endothelial progenitor cells and increased expression of VEGF).
4. Decreases pain at wound sites.
Strategy
1. Add to current estate of patents.
2. Gain opinion leader support from leading institutions.
3. Get FDA AOK for specific indication of uses and reimbursement.
4. Grow to over $100 million in annual sales.
5. Auction company off to highest bidder on May 11th, 2018.
Management Team – A mix of proven medtech industry veterans and eye scientists and clinicians. Microcurrent therapy leaders.
6 focused executives on management team. Over 70 business mentors and board members
Business Model
Sell direct in the USA to physicians and hospitals. Sell through distributors overseas. Get opinion leaders to present data at major meetings. Attend over 20 cardiovascular meetings a year with showcase. Publish data.
Market for dry age-related macular degeneration
- The current number of patients in the US and Europe is estimated to amount to 20 million.
- Aging populations in the western world will lead to a significant increase in numbers. In 2020
,about 30 million people are predicted to suffer from dry AMD. - The number of patients with wet AMD is comparable with the number of patients suffering from advanced dry AMD.
Competition
Dry Macular Degeneration
Although there are still very few development projects for the treatment of dry AMD, there is a handful of projects in advanced clinical stages of development.
- Alimera Sciences glucocorticoid “Fluocinolone Acetonide” (Ph II)
- Anti-Amyloid beta antibodies from
Alzheimers research of GlaxoSmithKline and Pfizer (PhII and PhI) - Alexion pharmaceuticals C5 inhibiting antibody (Ph II)
- Acucela’s visual cycle modulator ACU 4429 (Ph II)
- Neurotech’s implant with genetically modified human cells (Ph II)
- Stem Cell approaches by ACT (embryonic) and Stem Cells Inc. (central nervous system stem cells) (Ph I / II)
MacuClear’s and Succampos drugs for the improvement ofcoroidal blood flow (Ph II / III)
Wet Macular Degeneration
•
Note – Investment in this startup is only possible through the Cal-X Stars Business Accelerator, Inc. 506c Offering at this time. Please read completely the Private Placement Memorandum (PPM) via the link below and see all the warnings posted below and within the PPM. This offering is limited to accredited investors with > $1 million in assets or income > $200K a year or $300K as a couple past two years in a row.
ALL Cal-X Stars Business Accelerator, Inc. investors must verify their accredited status via Crowdentials, EarlyIQ or Healthiosxchange.
CIRCULATION on microcurrent improvement of blood flow – Click Here
Stimulator of choice for EyeCell studies

• The securities are being offered in reliance on an exemption from the registration requirements of the Securities Act and are not required to comply with specific disclosure requirements that apply to registration under the Securities Act;
• The Commission has not passed upon the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials;
• The securities are subject to legal restrictions on transfer and resale and investors should not assume they will be able to resell their securities; and
• Investing in securities involves substantial high risk, and investors should be able to bear the loss of their investment
RISK WARNING: The Cal-X Stars Business Accelerator, Inc. portfolio of innovations and startup companies are all early stage. The investment risk is very high and investors should be in
Note – New 506c regulations allow general advertising and up to 2000 shareholders while remaining private – http://www.sec.gov/rules/final/2013/33-9415.pdf
IMPORTANT LEGENDS (ANY POTENTIAL INVESTOR PLEASE READ)
Securities offered under Rule 506(c) may be purchased only by accredited investors = persons with > $1 million in assets excluding their home and vehicles or
Cal-X Stars Business Accelerator, Inc. develops
• The securities are being offered in reliance on an exemption from the registration requirements of the 150Securities Act and are not required to comply with specific disclosure requirements under the Securities Act; the Commission has not passed upon the merits of or given its approval to the securities, the terms of the offering, or the accuracy or completeness of any offering materials; the securities are subject to legal restrictions on transfer and resale and investors should not assume they will be able to resell their securities; and investing in securities involves risk and purchasers should be able to bear the loss of the entire investment. Private funds would be required to include a legend informing investors that the funds are not subject to the protections of the Investment Company Act.
